Solutions
Explanation-
The reaction of ether with the strong hydriodic acid (HI), causes the cleavage of the ether C-O bond, following the nucleophilic substitution reaction.
This cleavage further depends on the degree of alkyl group present in the ether as the primary and the secondary alkyl ethers have less steric hindrance. Thus, it shows the SN2 mechanism.
In the primary and the secondary alkyl ethers, following the SN2 mechanism, due to less hindrance, the iodide attaches to the smaller alkyl group, whereas in the tertiary alkyl ether following the SN1 mechanism, the high stability of the tertiary carbocation the iodide attaches to it, and the smaller alkyl forms the alcohol.
Option 1-
So, in CH3 - CH2 - CH2 - CH2 - O - CH3, through the SN2 mechanism, having 1∘ alkyl group, on reaction with HI, it forms butanol and methyl iodide.
Option 2-
In 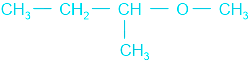 , having a 2∘ alkyl group, on reaction it forms methyl iodide and isobutyl alcohol.
, having a 2∘ alkyl group, on reaction it forms methyl iodide and isobutyl alcohol.
Option 3-
In the case of the tertiary alkyl ether due to steric hindrance, it follows the SN1 mechanism through the formation of the stable carbocation intermediate. So, in the compound given in option 3, on reaction we get tertiary- butyl iodide and methanol. As follows:

Option 4-
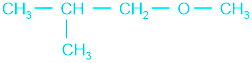 In reaction with HI, it forms 2-methyl propanol and methyl iodide.
In reaction with HI, it forms 2-methyl propanol and methyl iodide.
So option 3 is the correct answer.



 Get latest Exam Updates
Get latest Exam Updates 





 ×
×






