Solutions
Explanation:
Heat engine and its thermal efficiency:
Work (W) is high-grade energy that can be converted into heat (Q) as low-grade energy completely, but converting heat, a low-grade energy (Q) into work, high-grade energy (W) requires special devices which are known as heat engine.
The function of a heat engine is to produce work (Wnet) continuously at the expense of heat input (Q1) to it.
The fraction of heat input (Q1) that is converted to net-work output (Wnet) is a measure of performance of a heat engine and is called thermal efficiency.
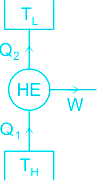
Q1 is the heat taken from the source (TH) and Q2 is the heat transferred to the sink (TL).
Net-work done by the engine, Wnet = Q1 - Q2.
Thermal efficiency of heat engine:
![]()
Mechanical efficiency
The ratio of brake power and indicated power is known as mechanical efficiency.



 Get latest Exam Updates
Get latest Exam Updates 





 ×
×






